3.11 Ions in liquid solutions
Many liquid substances undergo a process whereby their constituent molecules split into positively and negatively charged ion pairs, the positively-charge ion called a cation and the negatively-charged ion called an anion37 . Liquid ionic compounds38 split into ions completely or nearly completely, while only a small percentage of the molecules in a liquid covalent compound39 split into ions. The process of neutral molecules separating into ion pairs is called dissociation when it happens to ionic compounds, and ionization when it happens to covalent compounds.
Molten salt (NaCl) is an example of the former, while pure water (H2O) is an example of the latter. In liquid salt, practically every NaCl molecule splits up into an Na+ and Cl− ion pair, whereas with liquid water only a very small percentage of molecules split up into positively and negatively charged ions – most remain as whole H2O molecules. All the ions present in molten salt serve as electrical charge carriers, making molten salt a very good conductor of electricity. The scarcity of ions in a sample of pure water explains why it is often considered an insulator. In fact, the electrical conductivity of a liquid substance is the definitive test of whether it is an ionic or a covalent (“molecular”) substance.
The few water molecules that do ionize split into positive hydrogen ions40 (H+) and negative hydroxyl ions (OH−). At room temperature, the concentration of hydrogen and hydroxyl ions in a sample of pure water is quite small: a molarity of 10−7 M (moles of hydrogen ions per liter of solution) each.
Given the fact that pure water has a mass of 1 kilogram (1000 grams) per liter, and one mole of pure water has a mass of 18 grams, we must conclude that there are approximately 55.56 moles of water molecules in one liter (55.56 M). If only 10−7 moles of those molecules ionize at room temperature, that represents an extremely small percentage of the total:
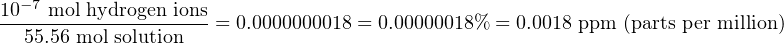
It is not difficult to see why pure water is such a poor conductor of electricity. With so few ions available to act as charge carriers, pure water is practically an insulator. The vast majority of water molecules remain un-ionized and therefore cannot transport electric charges from one point to another.
The molarity of both hydrogen and hydroxyl ions in a pure water sample increases with increasing temperature. For example, at 60 oC, the molarity of hydrogen and hydroxyl ions increases to 3.1 × 10−7 M, which is still only 0.0056 parts per million, but definitely larger than the concentration at room temperature (25 oC).
The electrical conductivity of water may be greatly enhanced by dissolving an ionic compound in it, such as table salt. When dissolved, the table salt molecules (NaCl) immediately dissociate into sodium cations (Na+) and chlorine anions (Cl−), becoming available as charge carriers for an electric current. In industry, we may exploit this relationship between electrical conductivity and ionic dissociation to detect the presence of ionic compounds in otherwise pure water.